June 4, 20201 min read, 290 words
Published: June 4, 2020 | 1 min read, 290 words
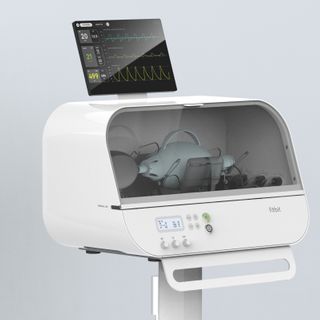
Fitbit has an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its Fitbit Flow emergency ventilator. The ventilator hardware is low-cost, and doesn’t require very much training or expertise to use, making it a good solution for deployment in ...
CRITIC REVIEWS
There don't seem to be any reviews yet.
PUBLIC REVIEWS
There don't seem to be any reviews yet.